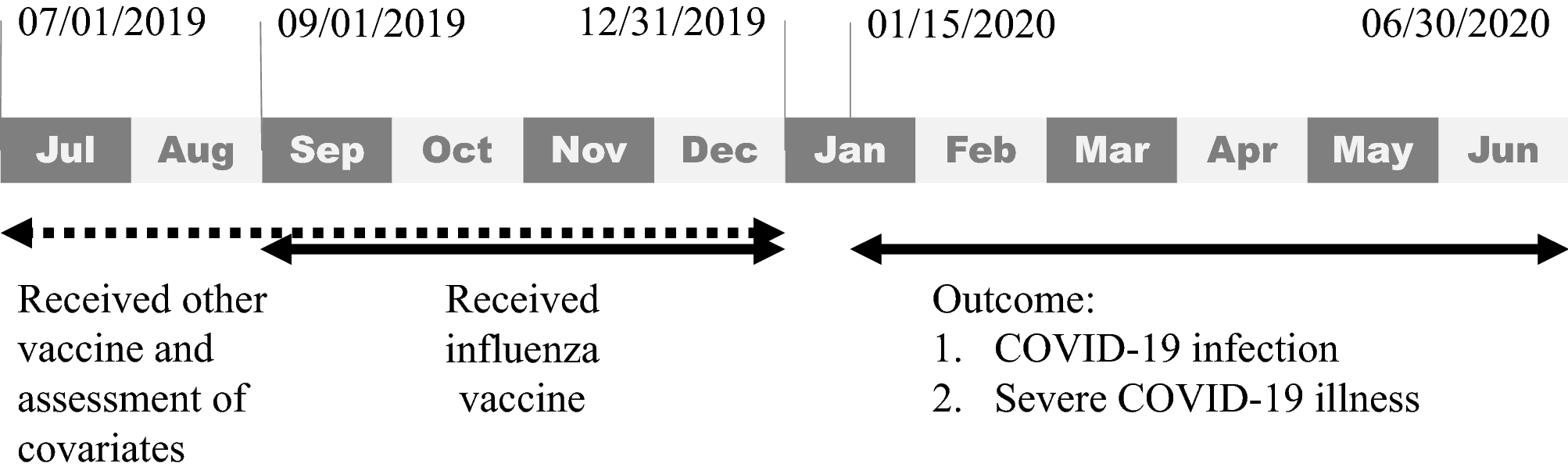
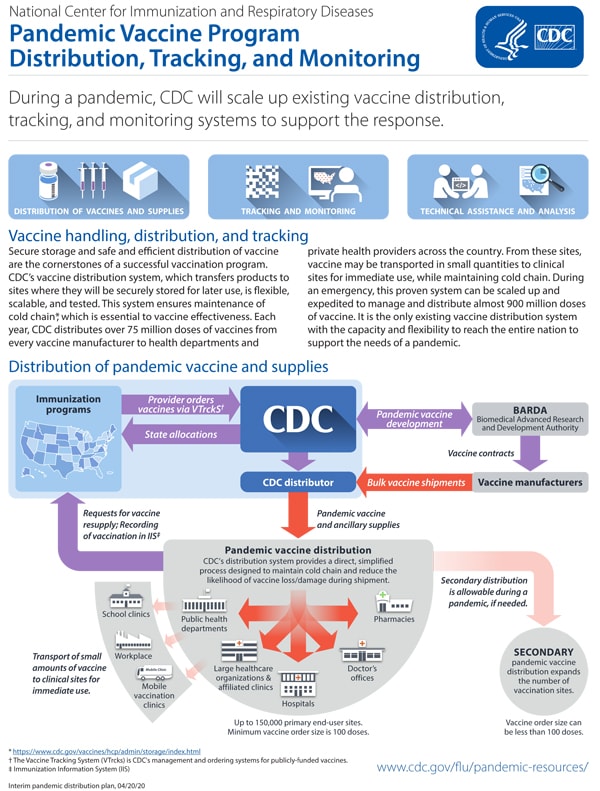
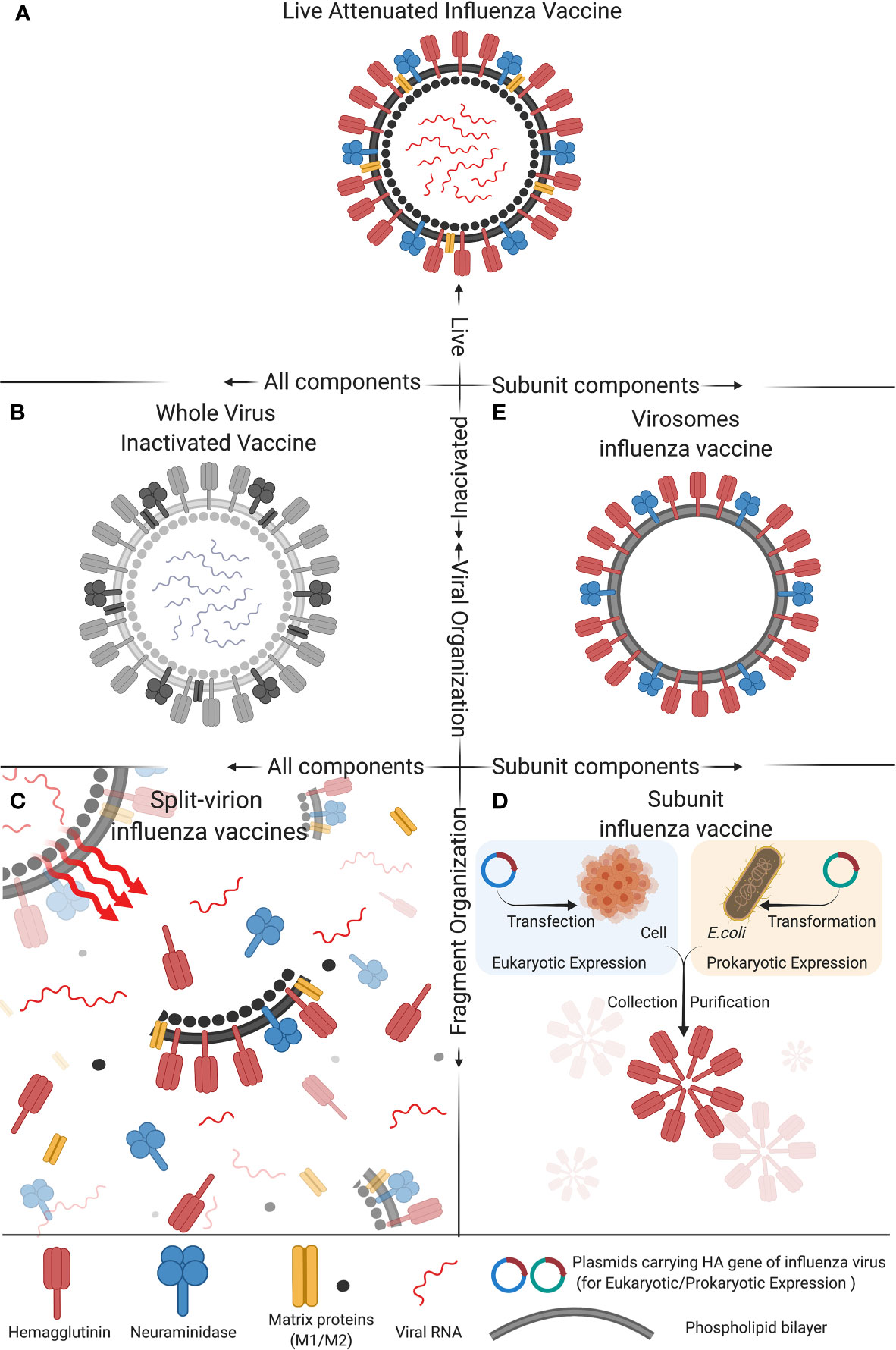
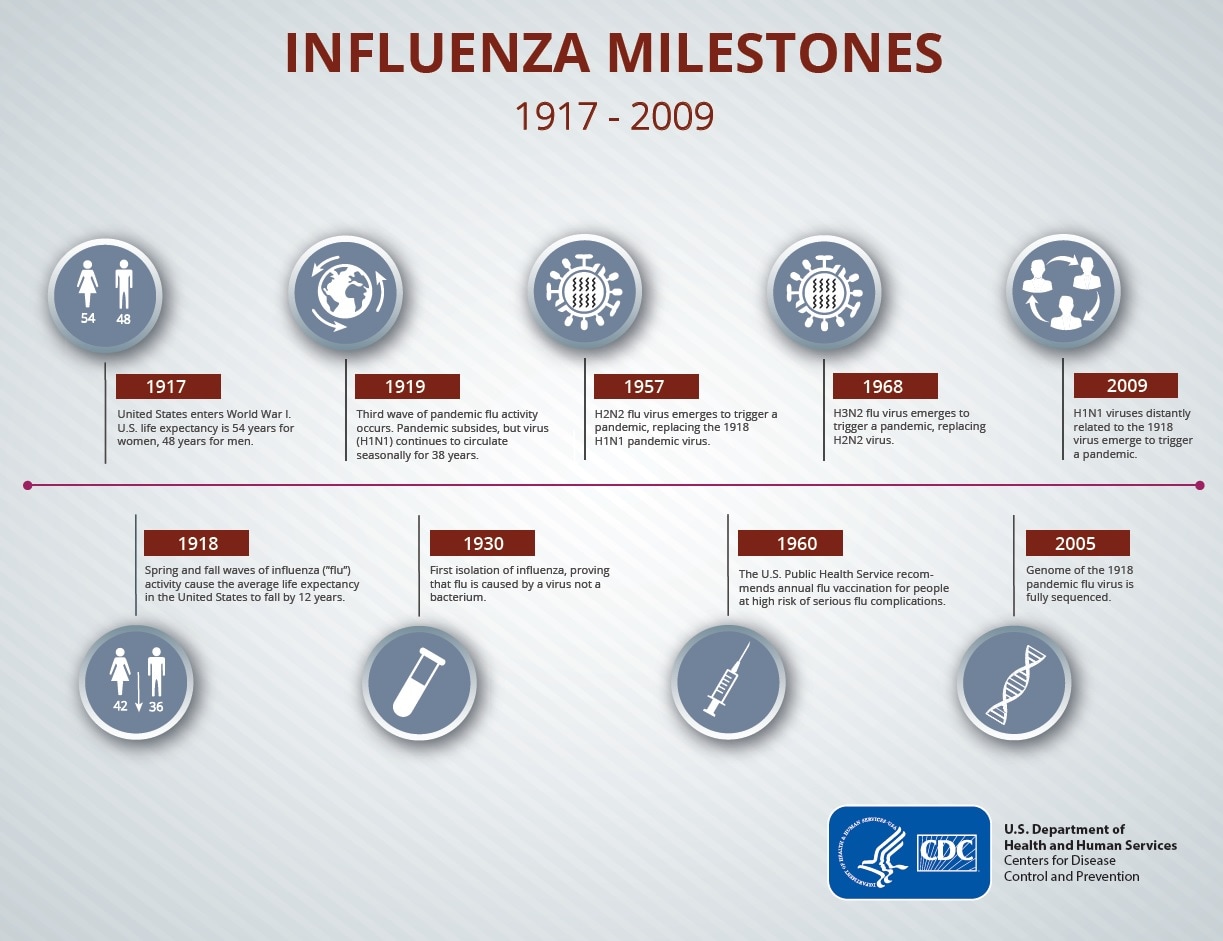
Collection of Influenza vaccine history ~ In 2014 CDC published a study to assess the occurrence of narcolepsy following. Any history of anaphylaxis to any prior vaccine.
as we know it recently has been searched by users around us, perhaps one of you personally. Individuals now are accustomed to using the net in gadgets to view video and image information for inspiration, and according to the title of the post I will talk about about Influenza Vaccine History A quadrivalent inactivated influenza vaccine was licensed in the United States in 2012 and a quadrivalent live virus nasal spray vaccine was licensed in 2013.
Influenza vaccine history
Collection of Influenza vaccine history ~ Additional recommendations for existing vaccines. Additional recommendations for existing vaccines. Additional recommendations for existing vaccines. Additional recommendations for existing vaccines. Or any known allergies to products contained in the investigational product. Or any known allergies to products contained in the investigational product. Or any known allergies to products contained in the investigational product. Or any known allergies to products contained in the investigational product. Out of the three types of influenza. Out of the three types of influenza. Out of the three types of influenza. Out of the three types of influenza.
Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Novel Influenza Vaccine Designs. Novel Influenza Vaccine Designs. Novel Influenza Vaccine Designs. Novel Influenza Vaccine Designs. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie.
Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. The 2021 Influenza Immunisation Programme start dates. The 2021 Influenza Immunisation Programme start dates. The 2021 Influenza Immunisation Programme start dates. The 2021 Influenza Immunisation Programme start dates. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic.
The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills.
Together through research and partnership we aim to move to a place of equity for all. Together through research and partnership we aim to move to a place of equity for all. Together through research and partnership we aim to move to a place of equity for all. Together through research and partnership we aim to move to a place of equity for all. Added this seasons Ovalbumin influenza vaccine table. Added this seasons Ovalbumin influenza vaccine table. Added this seasons Ovalbumin influenza vaccine table. Added this seasons Ovalbumin influenza vaccine table. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created.
The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. In 1978 the first trivalent flu vaccine was introduced. In 1978 the first trivalent flu vaccine was introduced. In 1978 the first trivalent flu vaccine was introduced. In 1978 the first trivalent flu vaccine was introduced. Influenza virus vaccine will not treat an active infection that has already developed in the body. Influenza virus vaccine will not treat an active infection that has already developed in the body. Influenza virus vaccine will not treat an active infection that has already developed in the body. Influenza virus vaccine will not treat an active infection that has already developed in the body.
A history of influenza Journal of Applied Microbiology. A history of influenza Journal of Applied Microbiology. A history of influenza Journal of Applied Microbiology. A history of influenza Journal of Applied Microbiology. Collected resources and information for influenza disease and vaccination. Collected resources and information for influenza disease and vaccination. Collected resources and information for influenza disease and vaccination. Collected resources and information for influenza disease and vaccination. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication.
Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. The programme for people aged under 65 years began on 17 May. The programme for people aged under 65 years began on 17 May. The programme for people aged under 65 years began on 17 May. The programme for people aged under 65 years began on 17 May. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season.
Intranasal influenza vaccine 2016 2000. Intranasal influenza vaccine 2016 2000. Intranasal influenza vaccine 2016 2000. Intranasal influenza vaccine 2016 2000. In response to the events in Europe CDC reviewed data from the US. In response to the events in Europe CDC reviewed data from the US. In response to the events in Europe CDC reviewed data from the US. In response to the events in Europe CDC reviewed data from the US. The 1918 influenza pandemic was the most severe pandemic in recent history. The 1918 influenza pandemic was the most severe pandemic in recent history. The 1918 influenza pandemic was the most severe pandemic in recent history. The 1918 influenza pandemic was the most severe pandemic in recent history.
Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza.
NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. The vaccination program was rushed yet plagued by delays and public relations problems. The vaccination program was rushed yet plagued by delays and public relations problems. The vaccination program was rushed yet plagued by delays and public relations problems. The vaccination program was rushed yet plagued by delays and public relations problems. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently.
The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919.
NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. Sophie Valtat et al. Sophie Valtat et al. Sophie Valtat et al. Sophie Valtat et al. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away.
This vaccine typically includes two influenza A strains and one influenza B strain. This vaccine typically includes two influenza A strains and one influenza B strain. This vaccine typically includes two influenza A strains and one influenza B strain. This vaccine typically includes two influenza A strains and one influenza B strain. The egg-based technology for producing influenza vaccine was created in the 1950s. The egg-based technology for producing influenza vaccine was created in the 1950s. The egg-based technology for producing influenza vaccine was created in the 1950s. The egg-based technology for producing influenza vaccine was created in the 1950s. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials.
HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. It was caused by an H1N1 virus with genes of avian origin. It was caused by an H1N1 virus with genes of avian origin. It was caused by an H1N1 virus with genes of avian origin. It was caused by an H1N1 virus with genes of avian origin. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine.
The influenza vaccination programme runs to 31 December 2021. The influenza vaccination programme runs to 31 December 2021. The influenza vaccination programme runs to 31 December 2021. The influenza vaccination programme runs to 31 December 2021. In 1918 influenza had neither treatment nor an effective vaccine. In 1918 influenza had neither treatment nor an effective vaccine. In 1918 influenza had neither treatment nor an effective vaccine. In 1918 influenza had neither treatment nor an effective vaccine. The first episode was possibly triggered. The first episode was possibly triggered. The first episode was possibly triggered. The first episode was possibly triggered.
Catch-up MMR Hepatitis B. Catch-up MMR Hepatitis B. Catch-up MMR Hepatitis B. Catch-up MMR Hepatitis B. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease.
The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. History diversity and their deep connection to the land. History diversity and their deep connection to the land. History diversity and their deep connection to the land. History diversity and their deep connection to the land.
CDC Vaccine Safety Efforts and Research. CDC Vaccine Safety Efforts and Research. CDC Vaccine Safety Efforts and Research. CDC Vaccine Safety Efforts and Research. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. HPV protecting against 9 types 2015 Discontinuation of vaccine. HPV protecting against 9 types 2015 Discontinuation of vaccine. HPV protecting against 9 types 2015 Discontinuation of vaccine. HPV protecting against 9 types 2015 Discontinuation of vaccine.
Influenza vaccine safety surveillance 2021. Influenza vaccine safety surveillance 2021. Influenza vaccine safety surveillance 2021. Influenza vaccine safety surveillance 2021. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. This update includes one new influenza virus vaccine code. This update includes one new influenza virus vaccine code. This update includes one new influenza virus vaccine code. This update includes one new influenza virus vaccine code.
Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. In 2012 the first quadrivalent flu vaccine was licensed in the United States. In 2012 the first quadrivalent flu vaccine was licensed in the United States. In 2012 the first quadrivalent flu vaccine was licensed in the United States. In 2012 the first quadrivalent flu vaccine was licensed in the United States.
There is limited data on the risk of recurrence of GBS in people where the first episode occurred within 6 weeks of influenza vaccination ie. There is limited data on the risk of recurrence of GBS in people where the first episode occurred within 6 weeks of influenza vaccination ie. There is limited data on the risk of recurrence of GBS in people where the first episode occurred within 6 weeks of influenza vaccination ie. There is limited data on the risk of recurrence of GBS in people where the first episode occurred within 6 weeks of influenza vaccination ie. Indeed most experts at the time thought that a bacterium caused influenza rather than a virus. Indeed most experts at the time thought that a bacterium caused influenza rather than a virus. Indeed most experts at the time thought that a bacterium caused influenza rather than a virus. Indeed most experts at the time thought that a bacterium caused influenza rather than a virus.

The Flu Shot Its History And Common Misconceptions Fortune
Source Image @ fortune.com

Influenza vaccine history | The Flu Shot Its History And Common Misconceptions Fortune
Collection of Influenza vaccine history ~ Additional recommendations for existing vaccines. Additional recommendations for existing vaccines. Additional recommendations for existing vaccines. Or any known allergies to products contained in the investigational product. Or any known allergies to products contained in the investigational product. Or any known allergies to products contained in the investigational product. Out of the three types of influenza. Out of the three types of influenza. Out of the three types of influenza.
Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Avian influenza known informally as avian flu or bird flu is a variety of influenza caused by viruses adapted to birds. Novel Influenza Vaccine Designs. Novel Influenza Vaccine Designs. Novel Influenza Vaccine Designs. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie. A history of GBS within 6 weeks of a previous flu vaccine a severe egg allergy ie.
Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. Meanwhile maximum military containment efforts succeeded unexpectedly in confining the new strain to the. The 2021 Influenza Immunisation Programme start dates. The 2021 Influenza Immunisation Programme start dates. The 2021 Influenza Immunisation Programme start dates. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic. In the US swine flu scare of 1976 President Gerald Ford was confronted with a potential swine flu pandemic.
The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. The type with the greatest risk is highly pathogenic avian influenza HPAIBird flu is similar to swine flu dog flu horse flu and human flu as an illness caused by strains of influenza viruses that have adapted to a specific host. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. Vaccine Adverse Event Reporting System VAERS and the Vaccine Safety Datalink VSD and found no indication of any association between US-licensed H1N1 or seasonal influenza vaccine and narcolepsy. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills. In addition the CR instructs MACs to modify existing editing to allow an influenza and a Pneumococcal Polysaccharide Vaccine PPV vaccination on the same date on separate roster bills.
Together through research and partnership we aim to move to a place of equity for all. Together through research and partnership we aim to move to a place of equity for all. Together through research and partnership we aim to move to a place of equity for all. Added this seasons Ovalbumin influenza vaccine table. Added this seasons Ovalbumin influenza vaccine table. Added this seasons Ovalbumin influenza vaccine table. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created. The need for a 2009 H1N1 influenza vaccine became apparent after the strains for the seasonal flu vaccine had already been selected so that a separate vaccine was created.
The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. The Ministry asks vaccinators to focus on immunising people eligible for a funded vaccine. In 1978 the first trivalent flu vaccine was introduced. In 1978 the first trivalent flu vaccine was introduced. In 1978 the first trivalent flu vaccine was introduced. Influenza virus vaccine will not treat an active infection that has already developed in the body. Influenza virus vaccine will not treat an active infection that has already developed in the body. Influenza virus vaccine will not treat an active infection that has already developed in the body.
A history of influenza Journal of Applied Microbiology. A history of influenza Journal of Applied Microbiology. A history of influenza Journal of Applied Microbiology. Collected resources and information for influenza disease and vaccination. Collected resources and information for influenza disease and vaccination. Collected resources and information for influenza disease and vaccination. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication. History of Guillain-Barré Syndrome whose first episode occurred within 6 weeks of receiving an influenza vaccine cancer immuno-oncology therapies checkpoint inhibitors The patient has been advised to consult with their treating oncologist about the risks and benefits of influenza vaccination other medical contraindication.
Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. Age distribution of cases and deaths during the 1889 influenza pandemic Vaccine. The programme for people aged under 65 years began on 17 May. The programme for people aged under 65 years began on 17 May. The programme for people aged under 65 years began on 17 May. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season. Added Ovalbumin content of the influenza vaccines for 2017 to 2018 flu season.
Intranasal influenza vaccine 2016 2000. Intranasal influenza vaccine 2016 2000. Intranasal influenza vaccine 2016 2000. In response to the events in Europe CDC reviewed data from the US. In response to the events in Europe CDC reviewed data from the US. In response to the events in Europe CDC reviewed data from the US. The 1918 influenza pandemic was the most severe pandemic in recent history. The 1918 influenza pandemic was the most severe pandemic in recent history. The 1918 influenza pandemic was the most severe pandemic in recent history.
Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. Information about the disease and vaccines for patients and parents P4208 Influenza vaccination of people with a history of egg allergy. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. The 2021 Influenza Immunisation Programme commenced on 14 April 2021 for people aged 65 and over. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza. Effective for claims processed with Dates of Service DOS on or after July 1 2020 influenza.
NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. NIAID Vaccine Research Center scientists have initiated Phase 12 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine called a DNA prime followed by a licensed seasonal influenza vaccine boost to improve the potency and durability of seasonal influenza. The vaccination program was rushed yet plagued by delays and public relations problems. The vaccination program was rushed yet plagued by delays and public relations problems. The vaccination program was rushed yet plagued by delays and public relations problems. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently. Scientists are also developing novel influenza vaccine platforms that could be produced more efficiently.
The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. The flu can cause serious illness especially in young children older adults and people with chronic health problems but anyone can become seriously ill from the flu virus. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Easy-to-use guide to post in your clinic to help determine if patients who report egg allergy should receive influenza vaccine. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919. Although there is not universal consensus regarding where the virus originated it spread worldwide during 1918-1919.
NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. NCIRS also acknowledges and pays respect to other Aboriginal and Torres Strait Islander nations from which our research staff and community are drawn. Sophie Valtat et al. Sophie Valtat et al. Sophie Valtat et al. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away. And though vaccines existed for several other diseases and a few useless and possibly harmful anti-flu vaccines were concocted an effective influenza vaccine was decades away.
This vaccine typically includes two influenza A strains and one influenza B strain. This vaccine typically includes two influenza A strains and one influenza B strain. This vaccine typically includes two influenza A strains and one influenza B strain. The egg-based technology for producing influenza vaccine was created in the 1950s. The egg-based technology for producing influenza vaccine was created in the 1950s. The egg-based technology for producing influenza vaccine was created in the 1950s. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials. NIAIDs Vaccine Research Center is developing DNA or gene-based vaccines against seasonal and pandemic influenza that have been tested in clinical trials.
HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. HPV 2011 to routinely vaccinate males intranasal influenza vaccine 2018 again recommended New versions of existing vaccines. It was caused by an H1N1 virus with genes of avian origin. It was caused by an H1N1 virus with genes of avian origin. It was caused by an H1N1 virus with genes of avian origin. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine. Currently a severe allergy to a vaccine component or history of a life threatening allergic reaction to a previous flu shot are the only CDC approved official contraindications medical reasons for not getting vaccinated to receiving influenza vaccine.
The influenza vaccination programme runs to 31 December 2021. The influenza vaccination programme runs to 31 December 2021. The influenza vaccination programme runs to 31 December 2021. In 1918 influenza had neither treatment nor an effective vaccine. In 1918 influenza had neither treatment nor an effective vaccine. In 1918 influenza had neither treatment nor an effective vaccine. The first episode was possibly triggered. The first episode was possibly triggered. The first episode was possibly triggered.
Catch-up MMR Hepatitis B. Catch-up MMR Hepatitis B. Catch-up MMR Hepatitis B. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. PHE advises individuals with a history of egg allergy can be immunised with either an egg free influenza vaccine if available or an influenza vaccine with an ovalbumin content less than 120 nanogramsmL facilities should be available to treat anaphylaxis. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease. Influenza virus vaccine works by exposing you to a small dose of the virus which helps your body to develop immunity to the disease.
The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. The influenza vaccine also called the flu vaccine is used to prevent infection caused by the influenza flu virus. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. Influenza vaccination is generally not recommended for people with a history of GBS whose first episode occurred with 6 weeks of receiving an influenza vaccine. History diversity and their deep connection to the land. History diversity and their deep connection to the land. History diversity and their deep connection to the land.
CDC Vaccine Safety Efforts and Research. CDC Vaccine Safety Efforts and Research. CDC Vaccine Safety Efforts and Research. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. The CDCs Advisory Committee on Immunization Practices has issued new guidelines for the prevention and control of seasonal influenza with vaccines for the 2021-2022 season. HPV protecting against 9 types 2015 Discontinuation of vaccine. HPV protecting against 9 types 2015 Discontinuation of vaccine. HPV protecting against 9 types 2015 Discontinuation of vaccine.
Influenza vaccine safety surveillance 2021. Influenza vaccine safety surveillance 2021. Influenza vaccine safety surveillance 2021. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. The first bivalent influenza vaccine was developed in 1942 as a response to the discovery of Influenza Type B. This update includes one new influenza virus vaccine code. This update includes one new influenza virus vaccine code. This update includes one new influenza virus vaccine code.
Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. Influenza virus vaccine is also available in a nasal spray form which is a live virus vaccine. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. History of a serious reaction to prior influenza vaccination or known allergy to constituents of influenza vaccines - including egg proteins - or polysorbate 80. In 2012 the first quadrivalent flu vaccine was licensed in the United States. In 2012 the first quadrivalent flu vaccine was licensed in the United States. In 2012 the first quadrivalent flu vaccine was licensed in the United States.
If you re searching for Influenza Vaccine History you've arrived at the right location. We have 20 images about influenza vaccine history adding pictures, photos, pictures, backgrounds, and more. In these page, we additionally provide variety of images available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
How Coronavirus Vaccine Development Compares To Other Shots In History
Source Image @ www.businessinsider.com
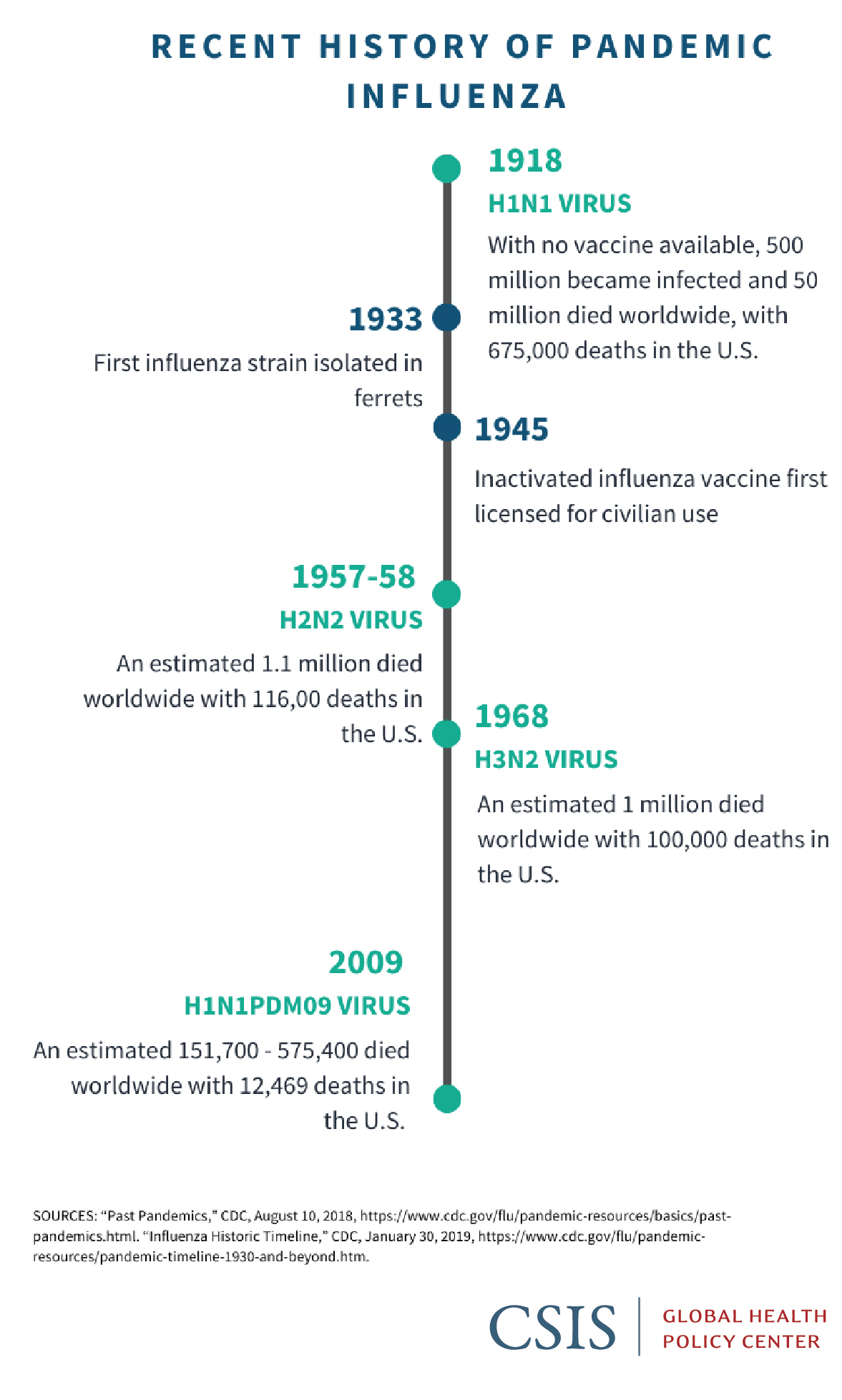
What Can The United States Do To Prevent Another Pandemic Commit To Modernizing Influenza Vaccines Center For Strategic And International Studies
Source Image @ www.csis.org

Timeline Of Current Influenza Vaccine Production Methods Schematic Download Scientific Diagram
Source Image @ www.researchgate.net
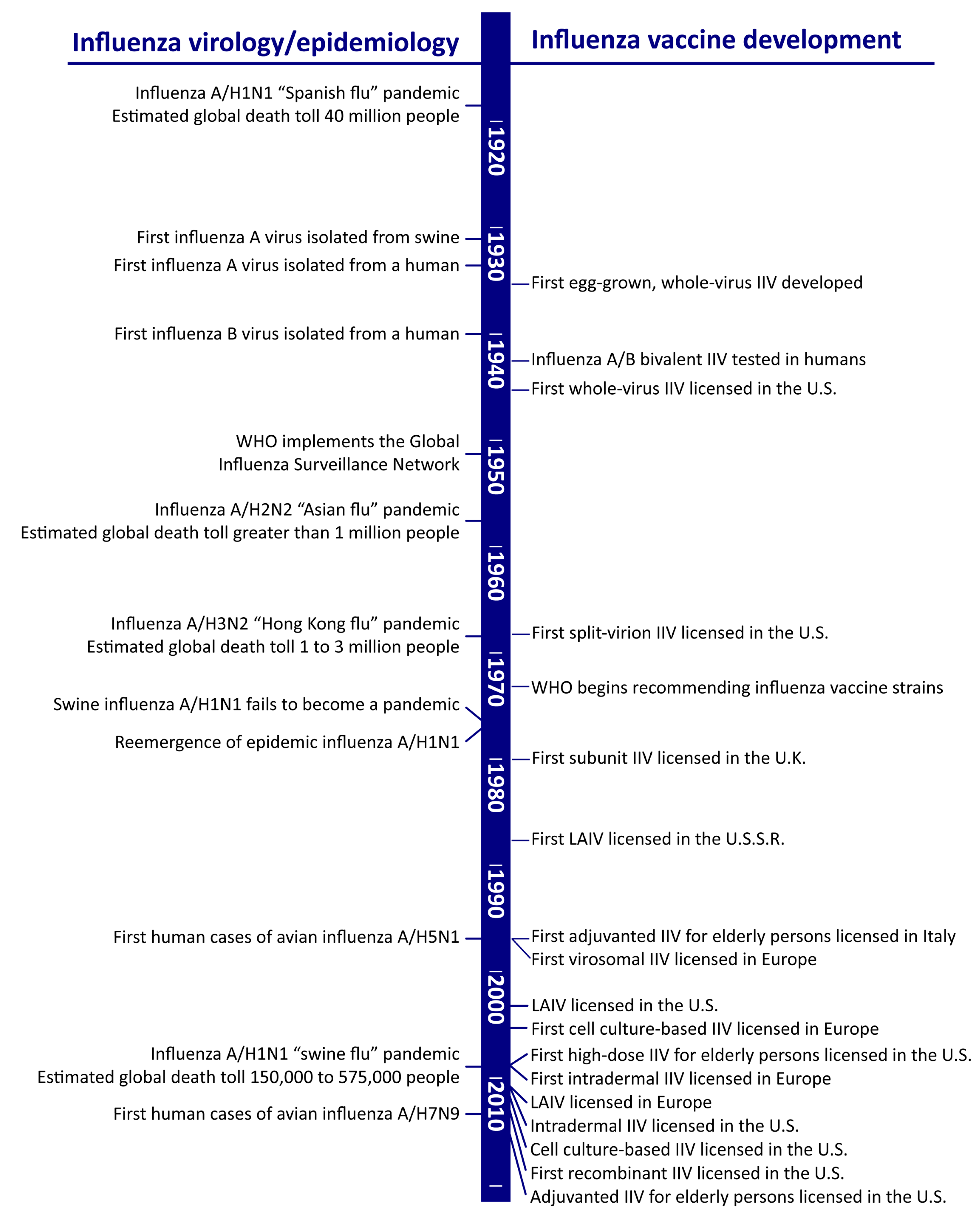
Vaccines Free Full Text The Future Of Influenza Vaccines A Historical And Clinical Perspective Html
Source Image @ www.mdpi.com